Patients
A new and innovative way to improve patients’ recoveries following colorectal surgery.
Professionals
An innovative solution for the protection of colorectal anastomosis.
Patient Benefits
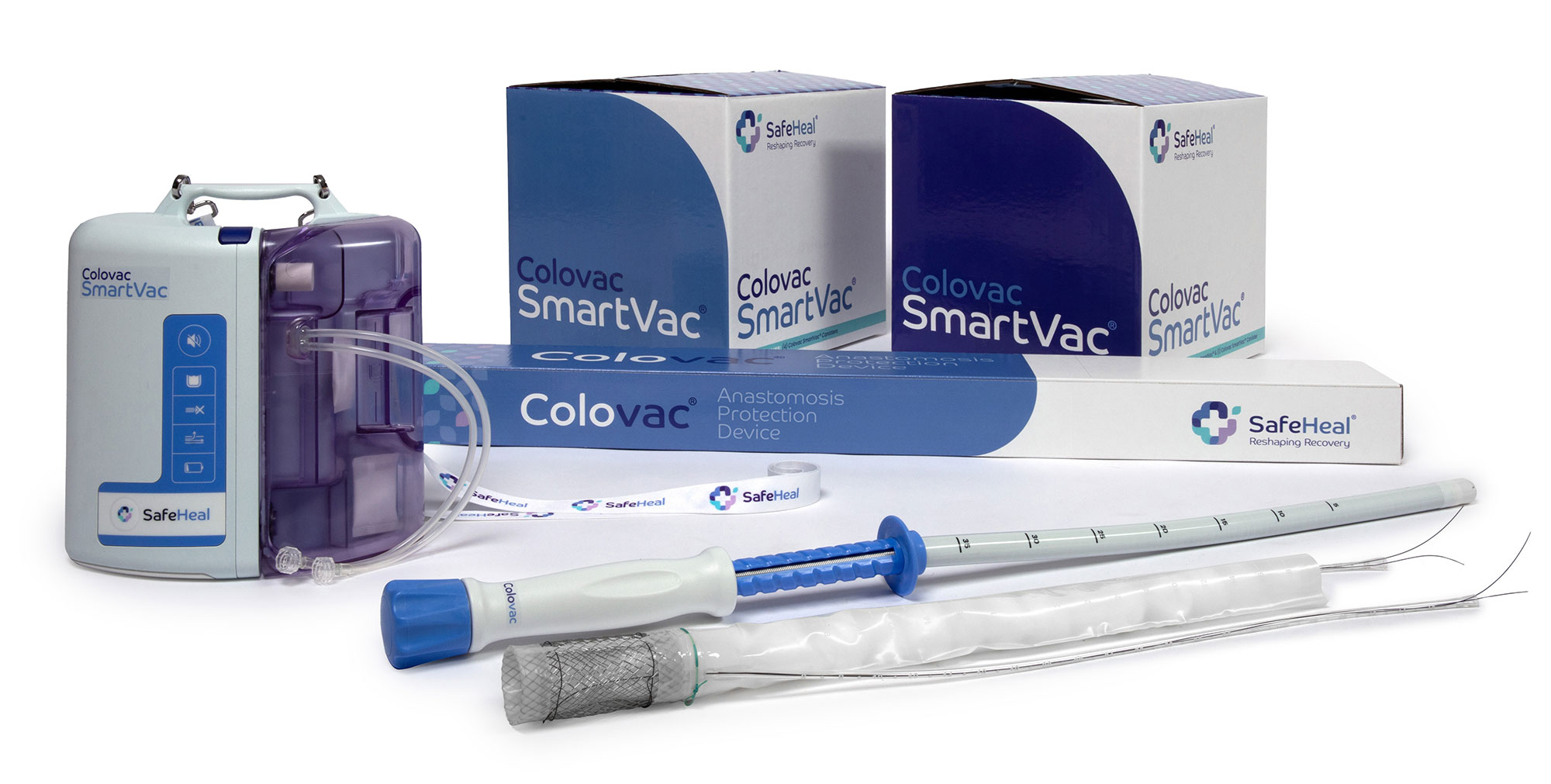
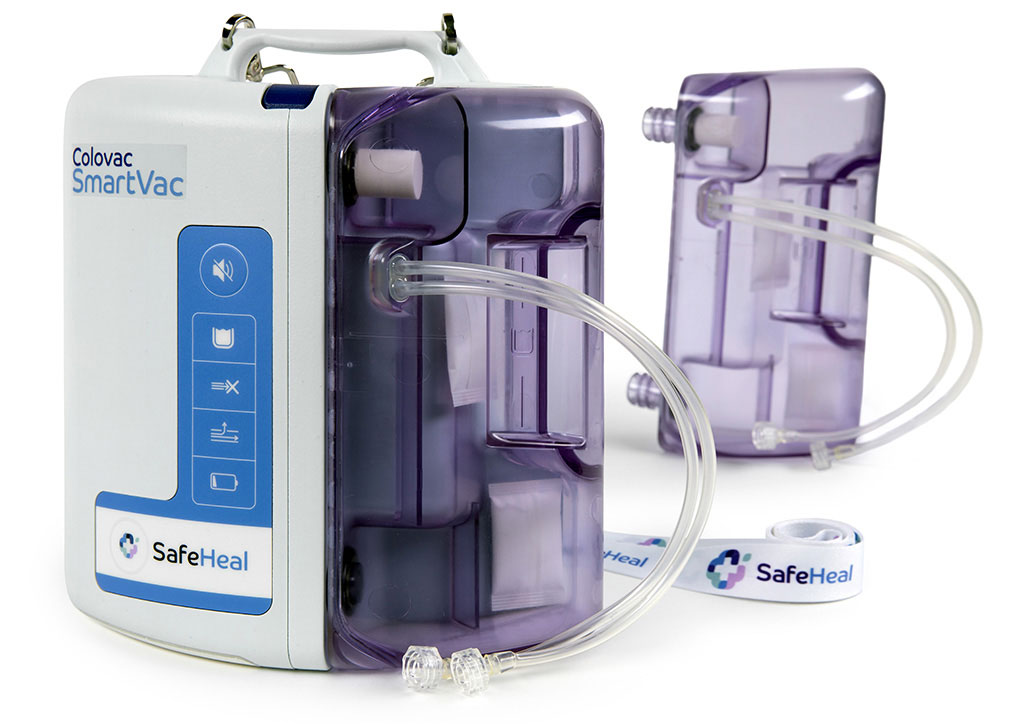
Product
Colovac is a colorectal anastomosis protection device designed to reduce the contact of fecal content at the anastomotic site following colorectal surgery.
The Company

Established in 2015 and based in France, SafeHeal is a clinical stage medical device manufacturer. Specializing in the development of anastomosis protection devices for colorectal surgery, SafeHeal’s premier device, Colovac, was invented to eliminate the need for diverting ostomies in patients undergoing colectomy.
Testimonials
Leading colorectal surgeons are talking about the benefits of Colovac.

Jorge Marcet, MD
Colon and Rectal Surgical Oncology at Tampa General Hospital Tampa, FL, USA
Read More"We are excited to be the first investigative site in the U. S. to enroll patients in SafeHeal’s clinical trial of the Colovac device. The aim of the SafeHeal study is to see if these patients can safely avoid an ostomy and avoid additional operations. Our initial experience with this device is positive and we are excited to be able to offer this cutting-edge technology to our patients as part of the study."

Prof. Jérémie Lefevre
General Surgery at Hôpital Saint-Antoine, Paris-France
Read More“The preliminary clinical trial results indicate that Colovac is an alternative to ileostomy, obviating the burden of stoma care for patients with a rectal cancer.”

Dr. Patricia Sylla
Colon and Rectal Surgery at Mount Sinai, New York-USA
Read More"There is a clinical need, Colovac’s approaching the problem from a different angle. For the patient, there is an immediate added value: avoiding an ostomy, it is an added value, no matter what."
Recent News
SafeHeal’s Jenny Gaffney to Address Medicare Reimbursement at The MedTech Conference 2025
Chris Richardson, CEO of SafeHeal, will be presenting an overview of the...
Read MoreSafeHeal® Receives European Marketing Approval Under MDR for Colovac® Anastomosis Protection Technology
SafeHeal announced that it has been granted European Union marketing approval for...
Read More- Chief Executive Officer Chris Richardson to Speak at LSI Emerging Medtech Summit: Click here to read the announcement
- SafeHeal appoints medical device industry veteran Chris Richardson as Chief Executive Officer: Click here to read the press release
1 Colovac is an investigational device limited by Federal (United States) law to investigational use. In the European Union, Colovac is exclusively used for clinical investigations. The Colovac device has not yet received regulatory approval, therefore the potential risks and benefits are not yet established.
V10.1_06162025






