Treatment of Colorectal Cancer
Colon cancer is the second most commonly occurring cancer in women, and the third most commonly occurring cancer in men. There were over 1.9 million new cases worldwide in 2020. Of these 1.9 million cases of cancer in the colon, approximately 500,000 are located in the rectum, the lowest segment of the colon. The following information is specific to the treatment of rectal cancer.
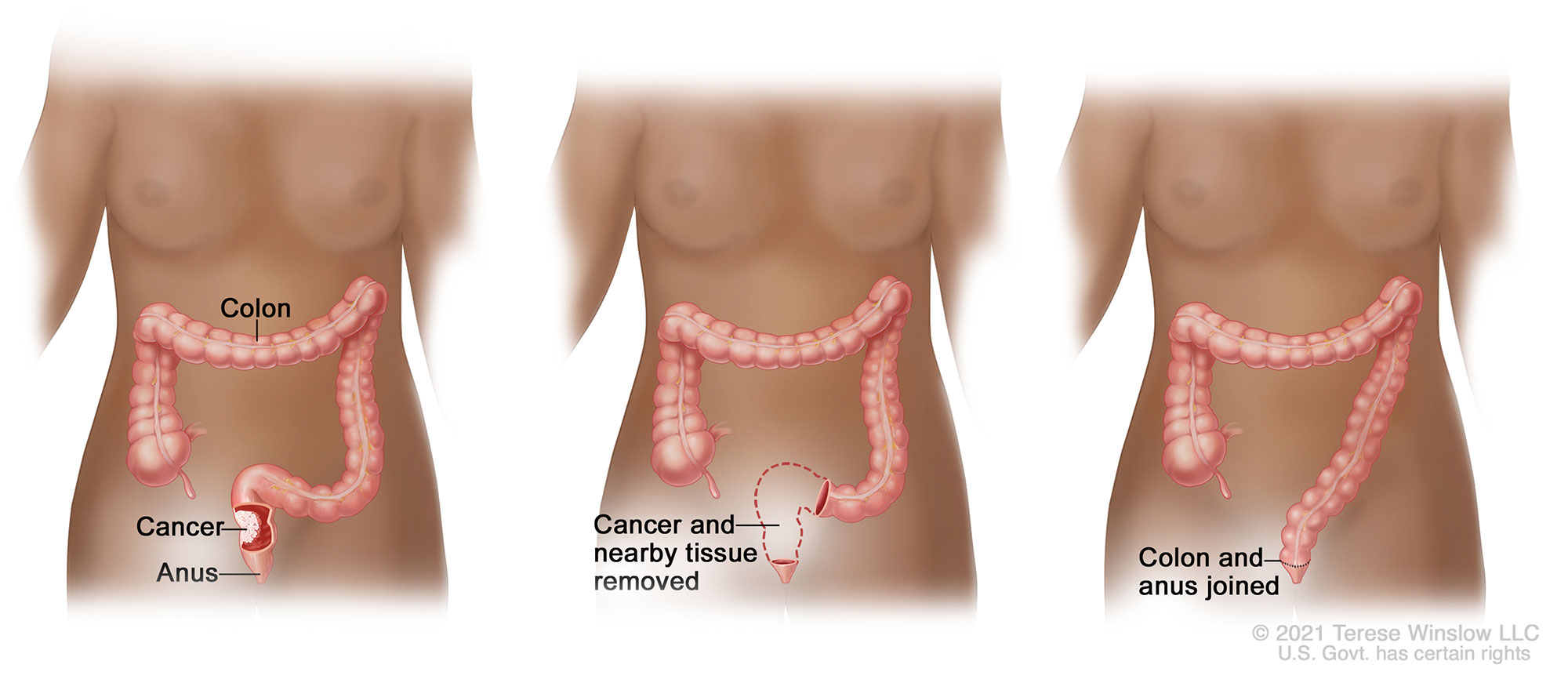
Treatment of rectal cancer most often involves radiation and chemotherapy followed by surgical removal of some or all of the rectum. The colon is then reconnected to the remaining rectum or anus with surgical staples or sutures; this connection is called an anastomosis.
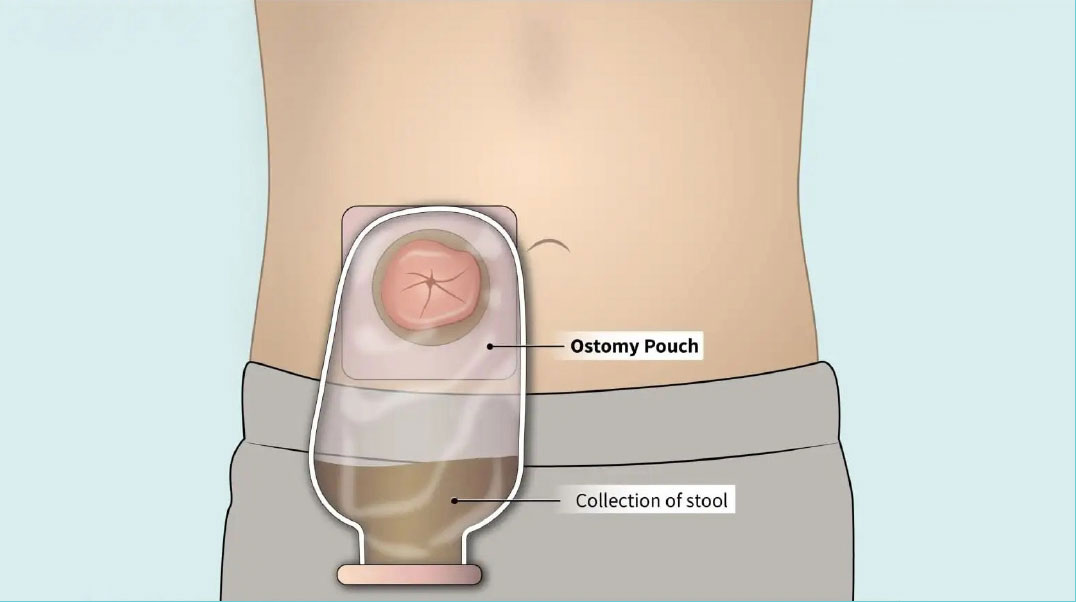
Because the risk of incomplete healing of the colon anastomosis is high, and the consequences could be dire, the surgeon may also create an ostomy (also called a stoma), which temporarily diverts the stool away from the healing anastomosis to the outside of the body and into an ostomy bag. Usually the ostomy is needed only until the rectum has healed, and then it can be reversed. If the entire rectum is removed, however, the ostomy may be permanent.
If the ostomy is intended to be temporary, the patient typically keeps the ostomy for approximately 2-9 months. The eventual reversal of the ostomy requires another operation, with a second hospital stay and recovery period.
Resection of the Rectum with Anastomosis
As with any surgery, complications can develop. While an ostomy procedure after rectal resection surgery is the current standard of care to provide protection to the healing anastomosis, specific risks are associated with the ostomy operation. The most common of these complications may include:
- Dehydration
- Irritation and inflammation of the skin around the ostomy
- A hernia at the site of the ostomy
Additionally, complications may develop from the secondary surgery 2-9 months later to reverse the ostomy.
In addition to physical complications, ostomy patients may experience an impact to their quality of life, due to:
- Social isolation
- Reduced physical activity and/or intimacy
- Extended recovery after cancer surgery
- Added expense of ostomy supplies
About Colovac1
Colovac is a colorectal anastomosis protection device intended to reduce the contact of fecal content at the anastomotic site following colorectal surgery. The device is placed at the time of the rectal resection surgery and is fully reversible. Colovac is designed to remain in place for 10 days, until the body’s natural healing and tissue repair processes are complete, after which it is removed during an endoscopic procedure similar to a colonoscopy, without the need for a second surgical intervention.
For the majority of patients, Colovac is expected to eliminate the need for a second surgery. This enables patients to resume normal activity after their cancer resection surgery without the stigma and complications associated with an ostomy procedure. Colovac is an investigational device, not currently available for sale. A multi-center clinical trial is underway to evaluate the safety and effectiveness of Colovac as an alternative to temporary diverting ostomy.

Rectal Cancer Treatment without Colovac
- Time to Full Recovery: 2-9 months
- 2 Surgeries, 2 Hospital Stays
- Risk of Complications
- 2 Surgeries Under Anesthesia
- Additional Incisions
- Ostomy Complications
- Risk of Permanent Ostomy
- Quality of Life Due to Ostomy Bag
- Social Isolation
- Impact to Physical Activity and Intimacy
- Added Expense of Ostomy Supplies
- Cost to Patient and Health Care System
- Multiple Surgeries and Hospital Stays
- Time to Full Recovery

Rectal Cancer Treatment with Colovac
- Time to Full Recovery: as little as 2 weeks
- Only 1 Surgery and Hospital Stay
- Risk of Complications
- Single Surgery under Anesthesia
- No Ostomy Complications
- Patient Satisfaction
- No Ostomy Bag, No Lifestyle Changes
- Faster Return to Normal Life
- Decreased Cost to Patient and Health Care System
- Single Surgery and Hospital Stay
- Reduced Time to Full Recovery
About the Colovac Clinical Study
Following pilot studies in Europe in which 70% of patients avoided an ostomy, SafeHeal is currently enrolling patients in a larger clinical study at up to 25 leading academic medical centers in the United States and Europe. The aim of the SafeHeal study is to assess the potential for patients with rectal cancer to safely avoid an ostomy with the use of a novel device, the Colovac colorectal anastomotic protection device.
- Colovac Potential Benefits
- Patients may avoid an ostomy
- No second surgery and hospital stay
- Faster return to normal life
Karen Zaghiyan, MD
Cedars-Sinai Medical Center
Justin Maykel, MD
UMASS Memorial Medical Center
The study consists of two phases; the first phase of the overall study is called SH-SOC23.
What is the SH-SOC23 clinical study?
- SH-SOC23 is a single arm, prospective, non-randomized, clinical study that will provide additional monitoring of anastomotic healing to otherwise Standard-of-Care treatment for rectal cancer. This study is currently enrolling patients in the U.S. and Europe.
What is the goal of SH-SOC23?
- This first phase of the two-part study will assess the rate of complications associated with the current standard-of-care surgery for rectal cancer. The information collected will be compared to the second phase of the study to be conducted later, which will involve the use of the Colovac device. The overall goal of the two-phase study is to validate the Colovac device as a safe and effective alternative to diverting ileostomy.
Why should a patient consider participating in SH-SOC23?
- Participation will provide patients with a more rigorous monitoring and follow-up of their post-surgical healing status, as compared to the current standard-of-care. Patients may experience a faster recovery with fewer complications due to:
- Early examination of the healing status of the anastomosis, which may minimize risks associated with leak
- Identification of other anastomotic complications which may be treated earlier, even before symptoms arise
- Early confirmation of a healed anastomosis may lead to an earlier stoma reversal (at surgeon discretion)
- The schedule of follow-up visits is consistent with the normal follow-up associated with cancer care
- There is no additional cost to the patient to participate in the SH-SOC23 study. Additionally, patients are compensated up to $1200 for completion of the required follow-up steps.
- Patients who participate are contributing to the body of evidence that may allow future patients to avoid an ostomy
The Institutional Review Board of each participating Clinical Study Site has assessed the risks associated with the SH-SOC23 protocol, and that appropriate steps have been taken to protect the rights and welfare of humans participating in the study.
You may be eligible to participate in the SH-SOC23 study if you;
- Are 18 years old or older
- Are undergoing a rectal cancer resection with a planned diverting ostomy
The following clinical partners are currently enrolling patients.
Please contact the surgeon investigator nearest you to discuss your eligibility for the SH-SOC23 study.
Clinical Partners:
United States
California
Cedars-Sinai Medical Center
Los Angeles, CA
CONTACT: Gayane Ovsepyan
Research & Education Program Coord.
Gayane.Ovsepyan@cshs.org
(310) 289-9224
Kaiser Permanente Los Angeles
Medical Center
Los Angeles, CA
 Elisabeth McLemore, MD (Bio)
Elisabeth McLemore, MD (Bio)CONTACT: Andrew Oh
Research Associate III
Andrew.Oh@kp.org
(323) 783-5475
Keck Medicine of USC
Los Angeles, CA
 Sang Lee, MD (Bio)
Sang Lee, MD (Bio)CONTACT: Valentina Rodina
Clinical Research Coordinator Supervisor
Valentina.Rodina@med.usc.edu
(323) 394-0756
Florida
Tampa General Hospital
Tampa, FL
 Jorge Marcet, MD (Bio)
Jorge Marcet, MD (Bio)CONTACT: Avennette Pinto
Clinical Research Associate
apinto@usf.edu
(813) 844-7948
University of Miami Medical Center
Miami, FL
 Nivedh Paluvoi, MD (Bio)
Nivedh Paluvoi, MD (Bio)CONTACT: Erick Ponce
Clinical Research Coordinator
exp554@miami.edu
(305) 243-7298
Illinois
Northwestern University
Chicago, IL
Massachusetts
Boston Medical Center
Boston, MA
 Jennifer Davids, MD (Bio)
Jennifer Davids, MD (Bio)CONTACT: Connor M. Roddy, MS
Research Coordinator
connor.roddy@bmc.org
(617) 414-6839
UMASS Memorial Medical Center
Worcester, MA
 Justin Maykel, MD (Bio)
Justin Maykel, MD (Bio)CONTACT: Samuel Feinberg
Clinical Research Coordinator II
Samuel.Feinberg1@umassmed.edu
(508) 334-4918
More
New York
Lenox Hill Hospital
New York, NY
 Joseph Martz, MD (Bio)
Joseph Martz, MD (Bio)CONTACT: Sasha Suarez, MD
Clinical Research Coordinator
SSuarez3@northwell.edu
(212) 434-4350
Maimonides Medical Center
New York, NY
 Rebecca Rhee, MD (Bio)
Rebecca Rhee, MD (Bio)CONTACT: Gene Sobol
Director, Performance Improvement
GSobol@maimonidesmed.org
(718) 283-7926
Mount Sinai Hospital
New York, NY
 Patricia Sylla, MD (Bio)
Patricia Sylla, MD (Bio)CONTACT: Roxanne Mistry
Clinical Research Coordinator I
Roxanne.Mistry@mountsinai.org
(212) 241-0492
New York Presbyterian-Weill Cornell Medical Center
New York, NY
 Dorna Jafari, MD (Bio)
Dorna Jafari, MD (Bio)CONTACT: Rohit Rasane
Clinical Research Specialist
rkr4004@med.cornell.edu
(646) 962-2789
North Shore University Hospital
Manhasset, NY
 Marc Greenwald, MD (Bio)
Marc Greenwald, MD (Bio)CONTACT: Tabetha Garver-Mosher
Clinical Research Coordinator
tgarvermosher@northwell.edu
Stony Brook Medicine
Stony Brook, NY
 Deborah Nagle, MD (Bio)
Deborah Nagle, MD (Bio)CONTACT: Victoria Boufis
Clinical Research Assistant
victoria.boufis@stonybrookmedicine.edu
(631) 444-8156
North Carolina
Novant Health
Clemmons, NC
 David Hiller, MD (Bio)
David Hiller, MD (Bio)CONTACT: Sabrina Bethea
Clinical Research Coordinator II
sabrina.bethea@novanthealth.org
(336) 718-6031
Pennsylvania
Geisinger Medical Center
Danville, PA
 Christopher Buzas, MD (Bio)
Christopher Buzas, MD (Bio)CONTACT: Kay Reiner
Clinical Research Coordinator III
kmreiner1@geisinger.edu
(570) 214-5421
Main Line Health System
Philadelphia, PA
 John Marks, MD (Bio)
John Marks, MD (Bio)CONTACT: Suraj Chetty
Clinical Research Assistant
chettys@mlhs.org
(716) 533-2971
Europe
Belgium
Antwerp University Hospital
Edegem, Belgium
France
Bordeaux Colorectal Institute Clinique Tivoli-Ducos
Bordeaux, France
CHU de Rouen
Rouen, France
Hôpital Saint-Antoine AP-HP
Paris, France
Hôpital Saint-Louis AP-HP
Paris, France
IHU Institut Hospitalo-Universitaire de Strasbourg
Strasbourg, France
Italy
Istituto Clinico Humanitas
Milan, Italy
1 Colovac is an Investigational Device, Limited by Federal (or United States) Law to Investigational Use.
V8.6_01232025







 Prof. Niels Komen
Prof. Niels Komen Prof. Quentin Denost
Prof. Quentin Denost Prof. Jean-Jacques Tuech
Prof. Jean-Jacques Tuech Prof. Jérémie Lefevre
Prof. Jérémie Lefevre Prof. Léon Maggiori
Prof. Léon Maggiori Prof. Didier Mutter
Prof. Didier Mutter Prof. Antonino Spinelli
Prof. Antonino Spinelli