Colovac1 is a colorectal anastomosis protection device intended to reduce the contact of fecal content at the anastomotic site following colorectal surgery. The device is placed at the time of the rectal resection surgery and is fully reversible. Colovac is designed to remain in place for 10 days, until the body’s natural healing and tissue repair processes are complete, after which it is removed during an endoscopic procedure similar to a colonoscopy, without the need for a second surgical intervention.
Preliminary clinical trial evidence supports Colovac’s effectiveness as an alternative to diverting stoma, improving and expediting patient recovery from colorectal surgery. Avoiding diverting stoma is a significant benefit for patients, surgeons, payors, and healthcare systems worldwide.
Colovac is an Investigational Device, Limited by Federal (or United States) Law to Investigational Use.
Timing
Colovac is designed to provide protection of the anastomosis during the critical initial 10-day anastomotic healing period.
No Ostomy
Colovac is designed to avoid the need for a diverting ostomy for the majority of patients who will not experience anastomotic complications.
Possible Reversal
Colovac is designed to allow for safe conversion to standard of care diverting ostomy for patients requiring extended anastomotic protection.
Clinical Benefits
Colovac is designed to address the #1 concern in colorectal surgery, which is protection of the healing anastomosis.
Every year, two million colorectal surgical resections are performed worldwide. In the healing phase after these surgeries, anastomotic leakage is a serious potential complication. To minimize the clinical impact of an anastomotic leak, for most low rectal resections a temporary diverting ostomy is created. Temporary diverting ostomy can lead to post-surgical complications, such as dehydration, parastomal hernia, bowel obstruction, and skin irritation. Temporary diverting ostomy is known to negatively impact patient satisfaction and quality of life, and a significant number of temporary diverting ostomies are never reversed, thus creating a permanent burden for patients.
Colovac has been studied as an alternative to diverting ostomy in multiple pilot clinical trials in France and Belgium. In these trials, Colovac was found to provide appropriate protection of the anastomosis from direct contact with feces during the implant period. Additionally, the placement and retrieval procedures of the Colovac device were found to be easy to perform with accurate device placement. Importantly, greater than 70% of participating patients successfully avoided a diverting ostomy.
By avoiding an ostomy, patients can return to their normal daily routine sooner, improving their overall quality of life.
Colovac will provide a local, temporary, less invasive protection of the anastomosis during the healing process:
How it Works
Colovac provides protection of the anastomosis during the critical 10-day post-operative healing stage, giving the majority of patients the benefit of avoiding a diverting ostomy.
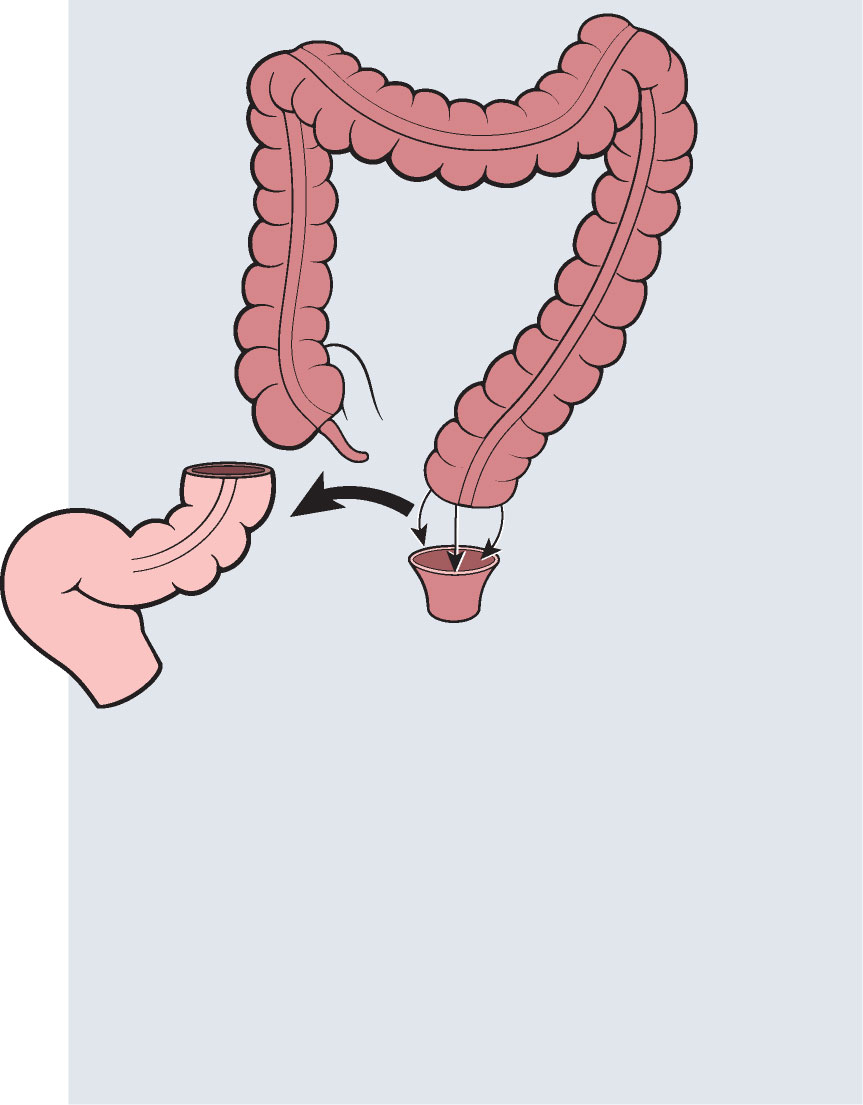
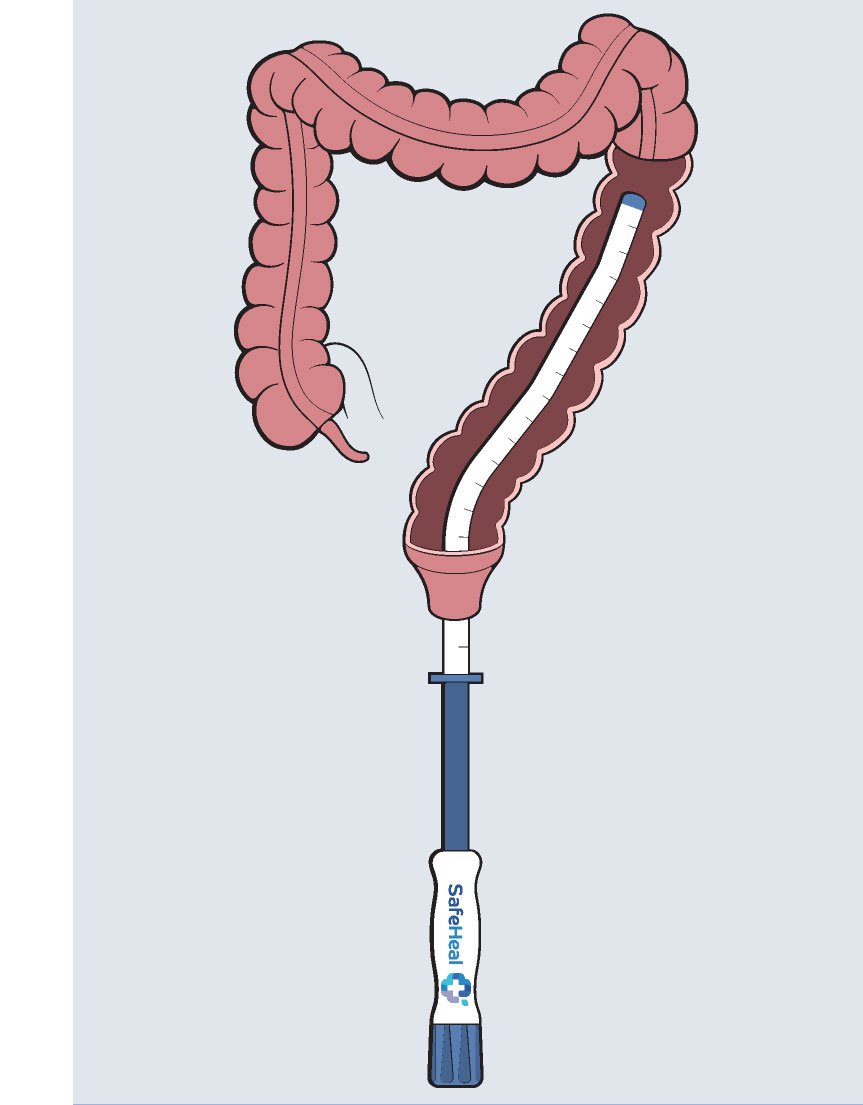
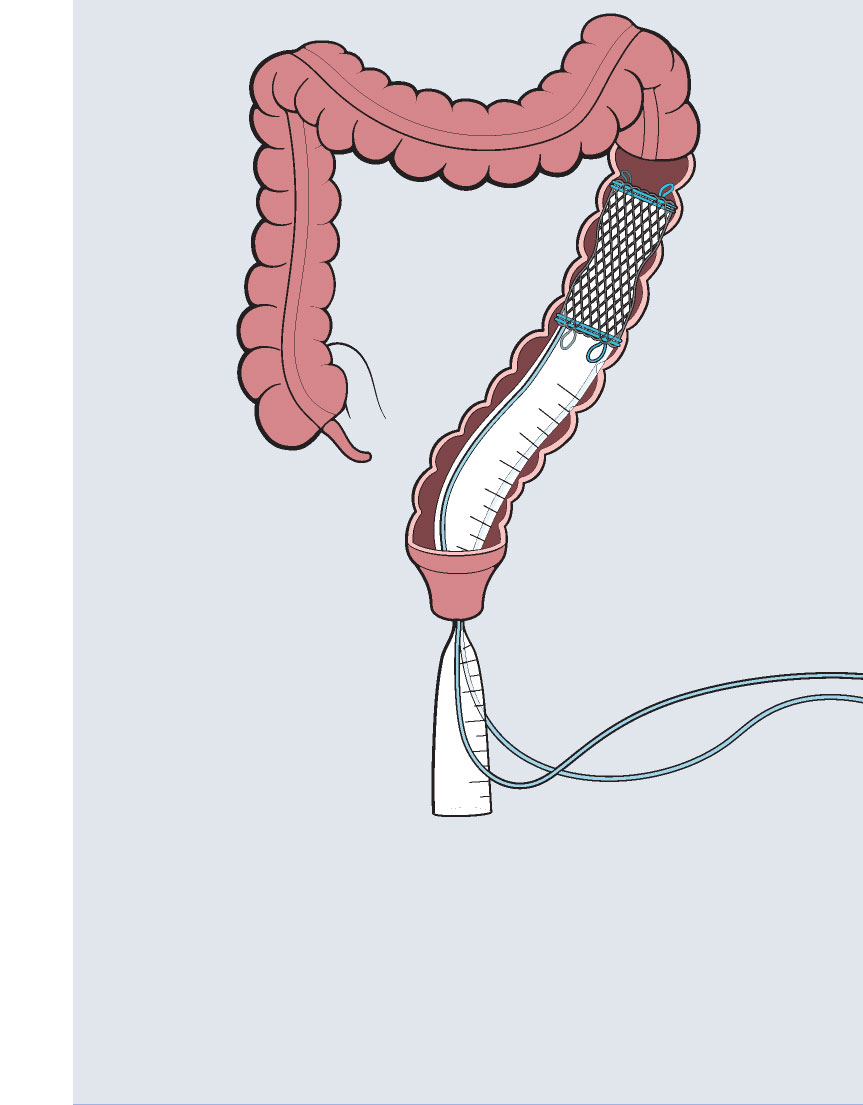
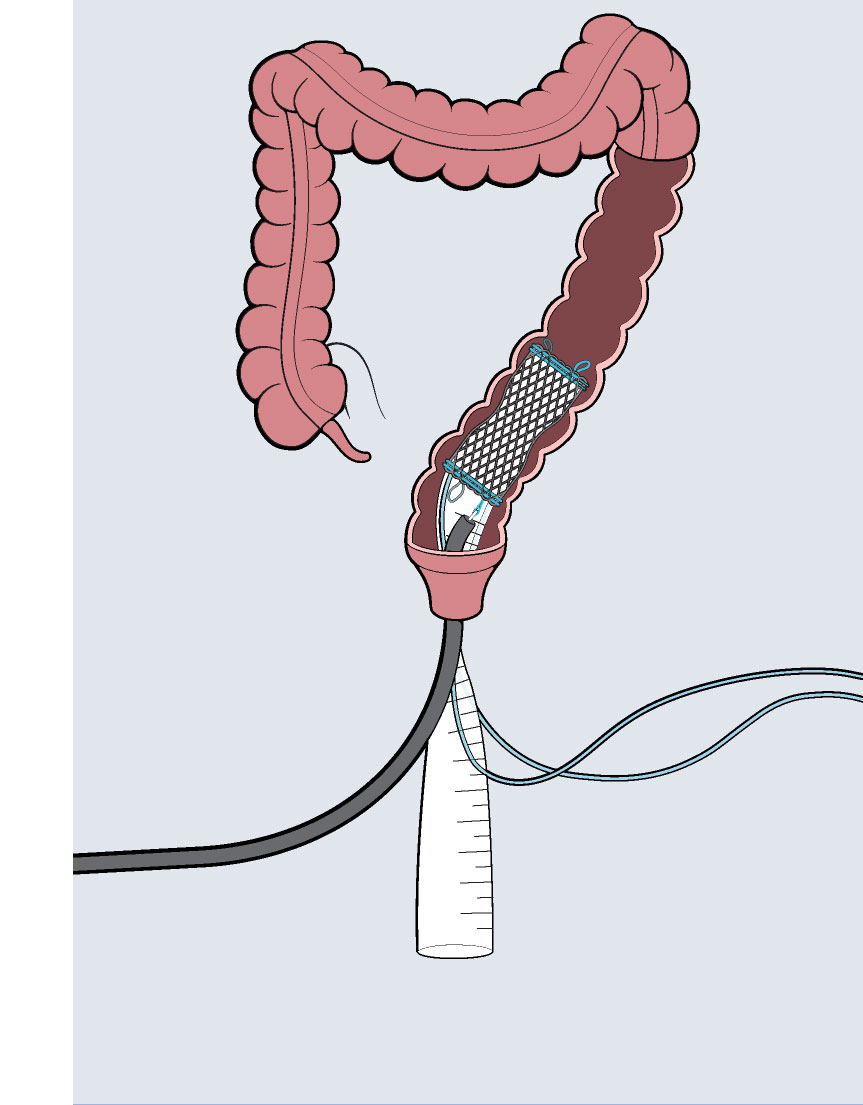
Colovac is comprised of a flexible bypass sheath that is anchored to the colon using a vacuum stent placed proximal to the anastomosis. The device is a minimally invasive, fully reversible internal bypass sheath that isolates the anastomosis from any contact with the fecal content during post-operative recovery, minimizing the sequelae of an anastomotic leakage (i.e. peritonitis).
Colovac Protection in 4 Steps
Surgery is performed as standard of care: open, laparoscopic or robotic approach.
Scientific Advisory Board

MD, The Mount Sinai Hospital, NY
Dr. Sylla is the Principal Investigator of SafeHeal’s multicenter pivotal study of Colovac. She has been a leader in minimally invasive approaches to the surgical treatment of colon and rectal cancer. Dr. Sylla pioneered taTME (transanal total mesorectal excision), a transanal endoscopic approach to perform rectal cancer surgery, and performed the first case in a human in 2009. She has since been actively engaged in training surgeons in this technique and organized clinical trials to evaluate the long-term outcomes of this approach.
Dr. Patricia Sylla
MD, The Mount Sinai Hospital, NY

MD, PhD, Amsterdam UMC
In 2006 he was appointed as a Professor in Surgery. His field of interest in patient care and research is the (minimal invasive) treatment of complex IBD, rectal cancer and complication surgery. Bemelman is a EBSQ certified colorectal surgeon (Bologna 2005), secretary of the European Crohn and Colitis organization and president elect of the European Society of Coloproctology. He guided more than 30 PhD students, has more than 460 peer reviewed papers and a H index of 57. He is currently working in the Amsterdam University Medical Center location Meibergdreef with his colorectal co-workers Roel Hompes, Pieter Tanis and Christianne Buskens.
Pr. Willem Bemelman
MD, PhD, Amsterdam UMC

MD, PHD, SAINT ANTOINE HOSPITAL, PARIS
Prof. Lefevre is the European Principal Investigator of SafeHeal’s multicenter pivotal study of Colovac. He is a digestive surgeon in the Saint-Antoine Hospital in Paris, one of the top academic departments of surgery in France. He is Professor of General surgery since 2015 at the Sorbonne University with a specialization in colorectal surgery. After one year as Gold Medal of Surgery of Paris, he completed his medical degree in 2008. He spent one year as Fellow in Oxford before his PhD in Physiology at the Pierre and Marie Curie University in 2012. His main research areas are colorectal cancers, especially the familial syndromes, and inflammatory bowel diseases.
Pr. Jérémie Lefevre
MD, PHD, SAINT ANTOINE HOSPITAL, PARIS
Testimonials
We are proud of our testimonials and comments we received from leading medical professionals.

Dr. Antonio D’Urso
Digestive surgery and bariatric at Hôpitaux Universitaires de Strasbourg, France
Read More"In order to always improve the quality of life of our patients, our hospital has agreed to be part of the clinical study. I am a huge advocate of the Colovac: it enables avoidance of diverting stoma, it is minimally invasive and fully reversible at any time."

Prof. Jérémie Lefevre
General Surgery at Hôpital Saint-Antoine, Paris-France
Read More“The preliminary clinical trial results indicate that Colovac is an alternative to ileostomy, obviating the burden of stoma care for patients with a rectal cancer.”

Dr. Patricia Sylla
Colon and Rectal Surgery at Mount Sinai, New York-USA
Read More"There is a clinical need, Colovac’s approaching the problem from a different angle. For the patient, there is an immediate added value: avoiding an ostomy, it is an added value, no matter what."
1 Colovac is an Investigational Device, Limited by Federal (or United States) Law to Investigational Use.
V8.6_01232025